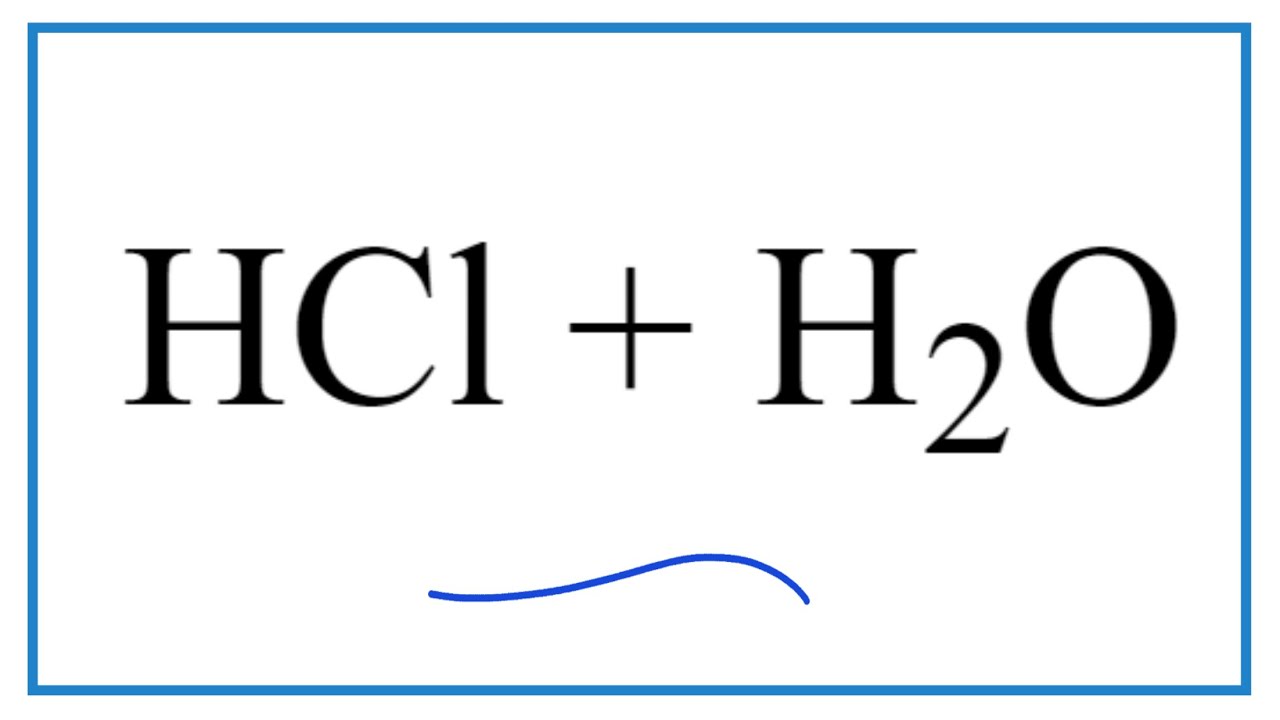Hydrochloric Acid and Water Dilution: A Comprehensive Guide
Combining hydrochloric acid and water is a common procedure in various scientific and industrial settings, from laboratory experiments to industrial processes. However, it's a process that must be approached with extreme caution due to the highly reactive nature of hydrochloric acid. Understanding the proper techniques is critical for safety and achieving the desired results. This guide provides a comprehensive overview of the process, covering essential safety precautions, chemical principles, and practical applications.
Diluting hydrochloric acid, or introducing hydrochloric acid to water, is not simply a matter of pouring the two liquids together. The highly exothermic nature of the reaction means that heat is generated when these substances combine. Adding water to concentrated hydrochloric acid can cause a violent reaction, potentially splashing the highly corrosive acid. Therefore, it's essential to always add the acid to the water slowly and steadily, allowing the solution to mix and the heat to dissipate gradually. This method minimizes the risk of splashing and ensures a controlled reaction.
Historically, hydrochloric acid, also known as muriatic acid, has been utilized for centuries. Early alchemists experimented with the substance, and its uses have evolved over time, spanning various industries. Today, combining hydrochloric acid with water is a foundational process in numerous applications. This underscores the importance of understanding the safe and proper procedures involved in this seemingly simple yet crucial chemical operation. Correct dilution techniques are paramount to prevent accidents and ensure the effectiveness of various chemical processes.
One of the primary issues associated with the incorrect addition of hydrochloric acid and water is the potential for dangerous splashing. Concentrated hydrochloric acid is highly corrosive, and contact with skin or eyes can cause severe burns. Inhaling the fumes generated during the reaction can also lead to respiratory irritation. These risks highlight the importance of following proper safety protocols, including wearing appropriate personal protective equipment (PPE) such as gloves, eye protection, and a lab coat, and working in a well-ventilated area. A fume hood is highly recommended when dealing with concentrated hydrochloric acid solutions.
The dilution of hydrochloric acid involves creating a less concentrated solution by adding water. This process is governed by the principle of molarity, which describes the concentration of a solution in terms of moles of solute per liter of solution. When water is added to hydrochloric acid, the number of moles of acid remains the same, but the volume of the solution increases, thus decreasing the molarity. For example, adding 1 liter of water to 1 liter of 1M hydrochloric acid creates 2 liters of 0.5M hydrochloric acid. Understanding this principle is essential for accurately preparing solutions of the desired concentration.
Advantages and Disadvantages of Diluting Hydrochloric Acid
| Advantages | Disadvantages |
|---|---|
| Achieve desired concentration for specific applications | Risk of splashing and chemical burns if not performed carefully |
| Reduce the corrosive strength for safer handling | Generation of heat and potentially harmful fumes |
| Enable controlled reactions in chemical processes | Requires proper safety precautions and equipment |
Best Practices for Diluting Hydrochloric Acid:
1. Always add acid to water, never the reverse.
2. Wear appropriate PPE, including gloves, eye protection, and a lab coat.
3. Work in a well-ventilated area or under a fume hood.
4. Add the acid slowly and steadily, stirring gently.
5. Allow the solution to cool before further handling.
FAQs:
1. What should I do if hydrochloric acid splashes on my skin? Immediately flush the affected area with copious amounts of water for at least 15 minutes.
2. What are the common uses of diluted hydrochloric acid? Cleaning, pH adjustment, and metal etching.
3. Can I dilute hydrochloric acid with other liquids besides water? Generally, no. Water is the standard solvent for dilution.
4. Why is it important to add acid to water and not vice versa? Adding water to acid can generate excessive heat and cause splashing.
5. What is the role of stirring during the dilution process? Stirring promotes even distribution of heat and prevents localized hotspots.
6. How do I store diluted hydrochloric acid? Store in a clearly labeled, tightly sealed container in a cool, dry place away from incompatible materials.
7. Where can I find more information about safe handling of hydrochloric acid? Consult safety data sheets (SDS) and chemical safety guidelines.
8. What are the symptoms of hydrochloric acid inhalation? Coughing, difficulty breathing, and burning sensation in the respiratory tract.
Tips and Tricks:
Use a graduated cylinder for accurate measurement of both acid and water. Keep a container of sodium bicarbonate nearby to neutralize any spills.
In conclusion, combining hydrochloric acid and water, specifically diluting hydrochloric acid, is a fundamental chemical process with wide-ranging applications. However, the highly reactive nature of hydrochloric acid requires strict adherence to safety guidelines. Always remember the crucial principle: add acid to water, never water to acid. Following the outlined safety procedures, understanding the chemical principles involved, and utilizing the best practices described in this guide will ensure safe and effective dilution of hydrochloric acid, preventing accidents and facilitating successful outcomes in various scientific and industrial endeavors. By prioritizing safety and employing the correct techniques, you can harness the power of hydrochloric acid solutions while mitigating the potential risks associated with this important chemical compound. Remember to consult safety data sheets and other resources for more detailed information and guidance specific to your application.
Unlock skip bo fun without spending a dime
Rock your denim the ultimate guide to knee high cut off jean shorts
Extreme trailers dallas texas your guide to heavy duty hauling