Unlocking Reactions: The Power of Potential Energy Diagrams in Chemistry
Ever wonder how chemists predict the outcome of reactions? They have a secret weapon: the potential energy diagram. These labeled charts, crucial in the field of chemistry, map out the energy landscape of a reaction, showing how energy changes as reactants transform into products.
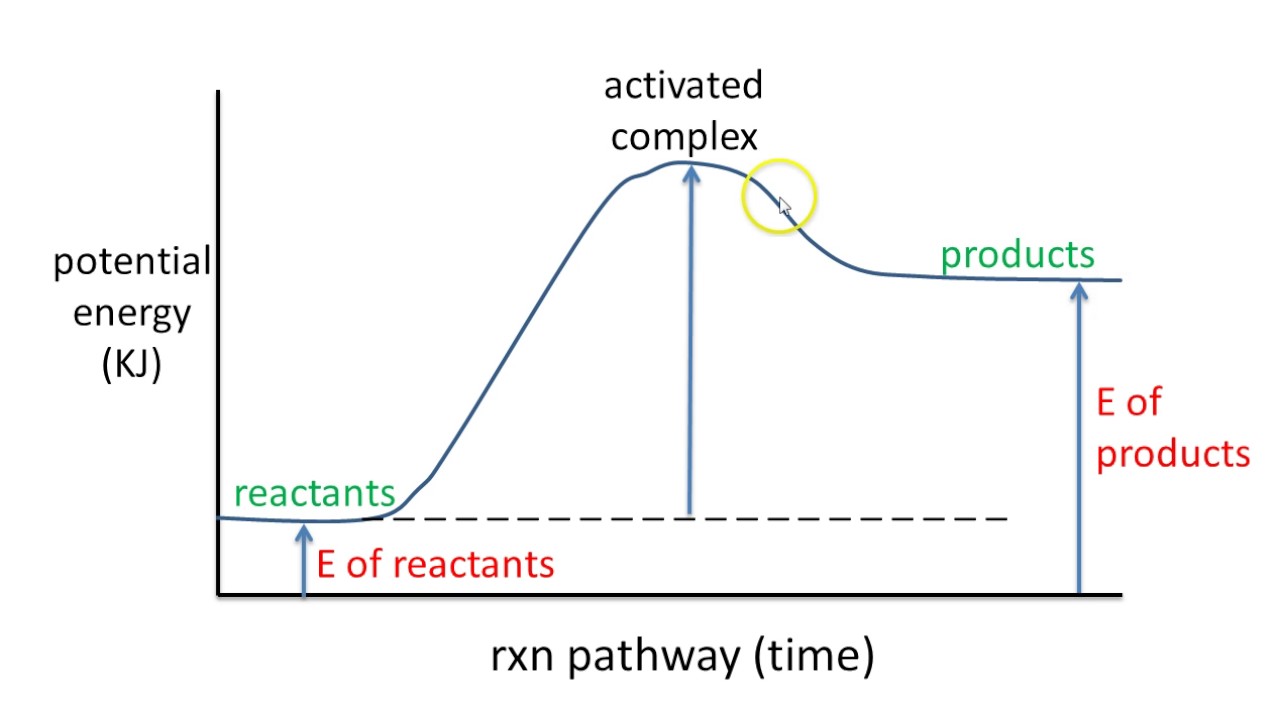
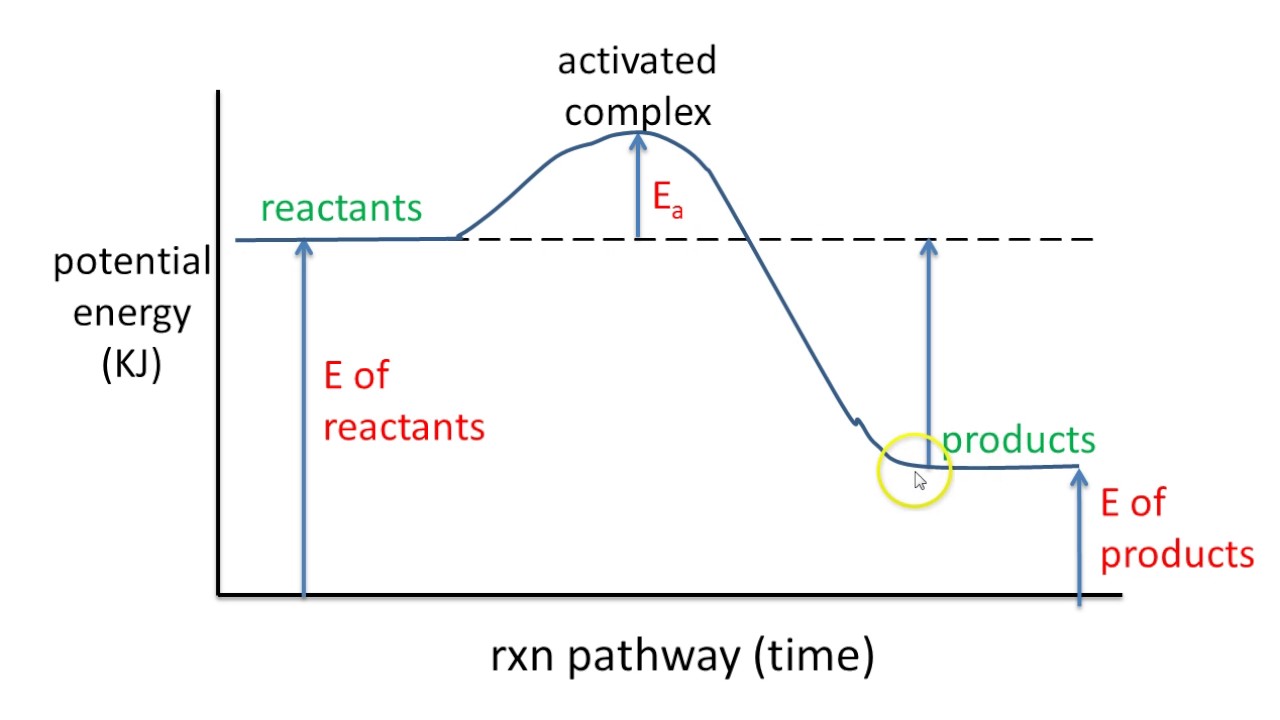
Imagine climbing a mountain. The peak represents the energy barrier a reaction must overcome. That's the activation energy. A potential energy diagram visually represents this "climb," providing key insights into reaction dynamics. It’s not just about the peak, though; the entire journey from the valley of reactants to the plateau of products is captured, revealing energy changes at each stage.
These diagrams aren't just theoretical doodles. They're essential tools for understanding reaction mechanisms, predicting reaction rates, and even designing catalysts. A labeled potential energy diagram in chemistry can tell you whether a reaction will be fast or slow, exothermic (releasing energy) or endothermic (absorbing energy).
The concept of potential energy diagrams emerged from the development of thermodynamics and chemical kinetics in the late 19th and early 20th centuries. Scientists needed a way to visualize the energy changes accompanying chemical transformations. These diagrams became a crucial bridge between theory and experiment, allowing chemists to interpret experimental data and refine their understanding of reactions.
One of the main issues addressed by these diagrams is understanding the activation energy barrier. Why do some reactions happen spontaneously while others require a hefty energy input? The diagram visually represents this barrier, clarifying why some reactions proceed readily at room temperature while others need a kick-start, like heat or a catalyst. This knowledge is fundamental to controlling and optimizing chemical reactions in countless applications, from industrial processes to drug development.
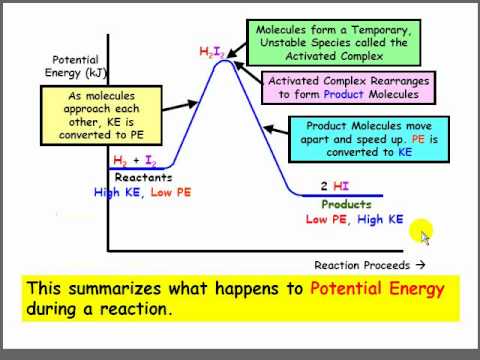
A potential energy diagram plots the potential energy of a chemical system as the reaction progresses. The x-axis represents the reaction coordinate (a measure of reaction progress), while the y-axis represents potential energy. For example, in the reaction A + B → C, the diagram would show how the potential energy changes as A and B interact and transform into C.
Benefits of using potential energy diagrams include: 1) Visualizing reaction mechanisms: The diagram shows the intermediate steps involved in a reaction. 2) Predicting reaction rates: The activation energy height indicates the reaction rate. A higher barrier means a slower reaction. 3) Understanding catalysis: Catalysts lower the activation energy, making reactions proceed faster. For instance, enzymes in our bodies act as biological catalysts, speeding up essential biochemical reactions.
To interpret a potential energy diagram, first identify the reactants and products on the diagram. Then, locate the activation energy – the difference in energy between the reactants and the highest point on the curve. Finally, determine whether the reaction is exothermic or endothermic by comparing the energy levels of reactants and products. An exothermic reaction has products at a lower energy level than reactants, while an endothermic reaction has products at a higher energy level.
Advantages and Disadvantages of Potential Energy Diagrams
| Advantages | Disadvantages |
|---|---|
| Visual representation of reaction progress | Simplified representation; doesn't show all details |
| Clarifies activation energy and reaction rates | Can be complex for multi-step reactions |
| Helps understand catalysis | Doesn't account for solvent effects in detail |
One real-world example is the combustion of methane. The potential energy diagram for this reaction shows a high activation energy, which is why methane doesn't spontaneously combust in air. However, once ignited, the reaction releases a significant amount of energy, as seen by the lower energy level of the products.
A common challenge is interpreting complex diagrams for multi-step reactions. The solution is to break down the overall reaction into individual steps and analyze each step’s potential energy diagram.
FAQ: What is activation energy? How does a catalyst work? What is the reaction coordinate? What is the difference between an exothermic and an endothermic reaction? How does temperature affect reaction rate? What is the transition state? How can I draw a potential energy diagram? What are the limitations of potential energy diagrams?
One trick for remembering the difference between exothermic and endothermic reactions is to think of "exo" as "exiting" (energy exits the system) and "endo" as "entering" (energy enters the system).
Potential energy diagrams are essential tools in chemistry for understanding reaction mechanisms and kinetics. They provide a visual representation of energy changes during a reaction, clarify the role of activation energy, and help us understand how catalysts work. By mastering the interpretation of these diagrams, we gain valuable insights into the fundamental principles governing chemical transformations. From designing more efficient industrial processes to developing new pharmaceuticals, understanding potential energy diagrams is critical for advancing our knowledge and manipulating chemical reactions to our benefit. Start exploring these powerful tools today and unlock a deeper understanding of the chemical world around you!
Navigating the jonesboro ar dhs office
Unlocking value rights issue offer letters
Upgrade your boats electrical system with a rocker switch panel